Photo used with courtesy by William Preston Bowling
Constituents of Concern (COCs)
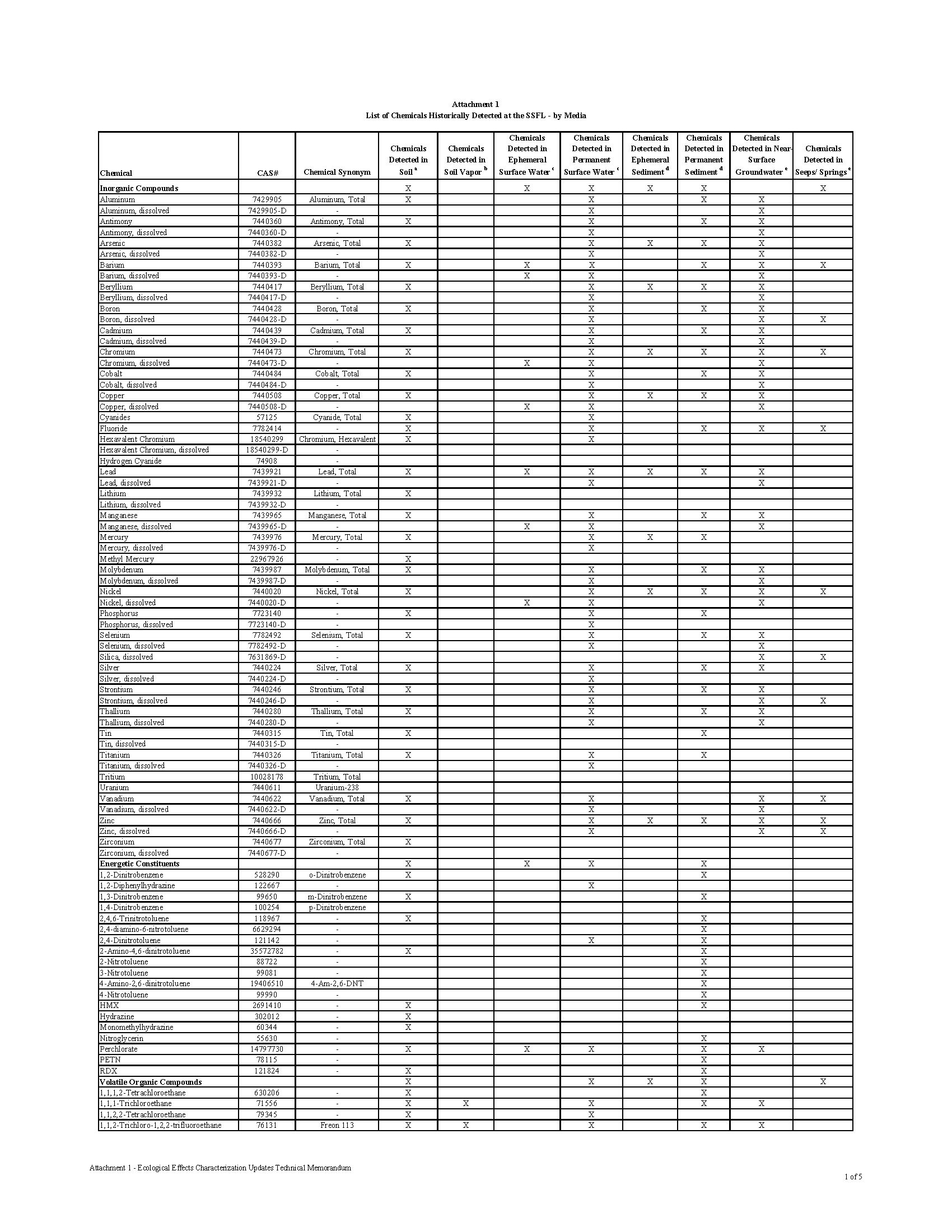
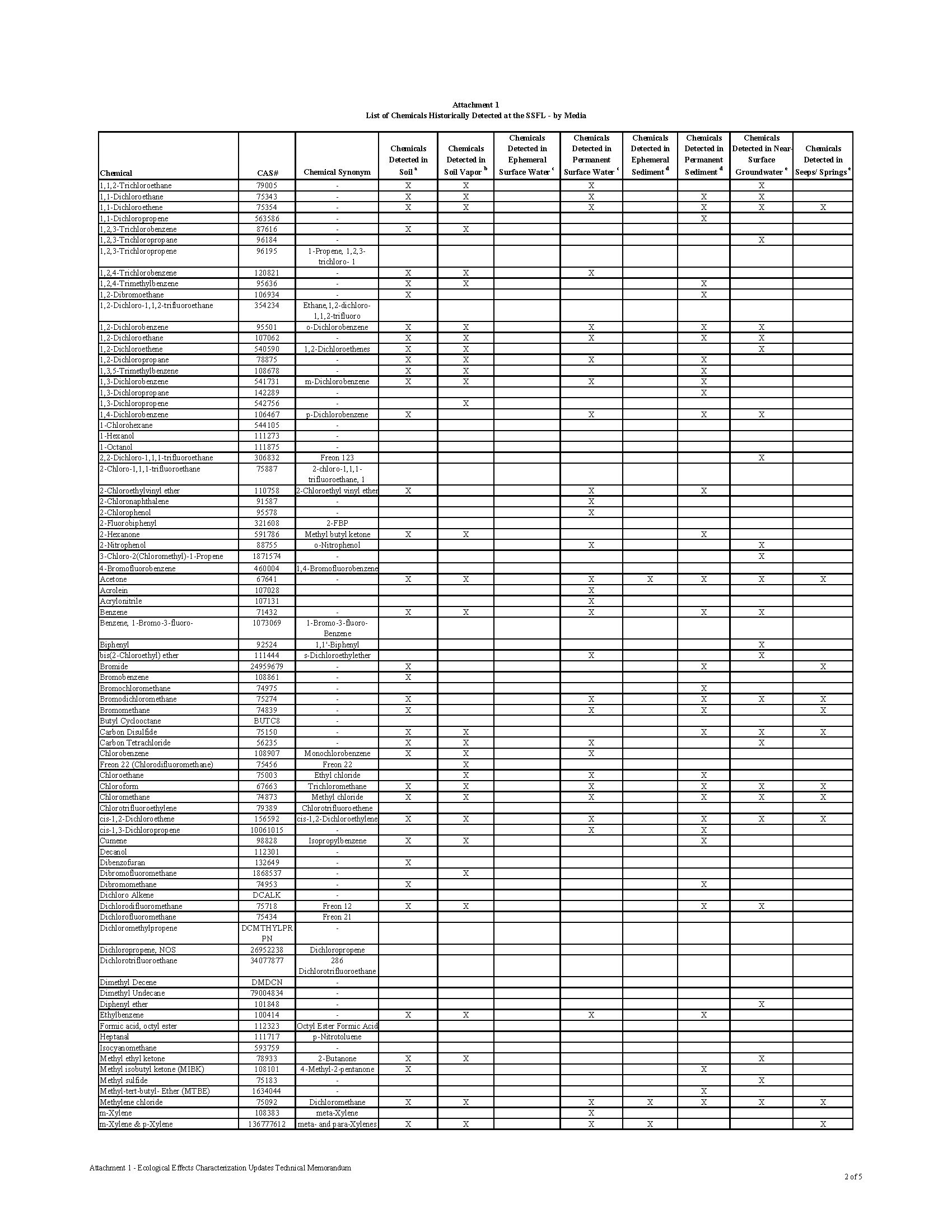
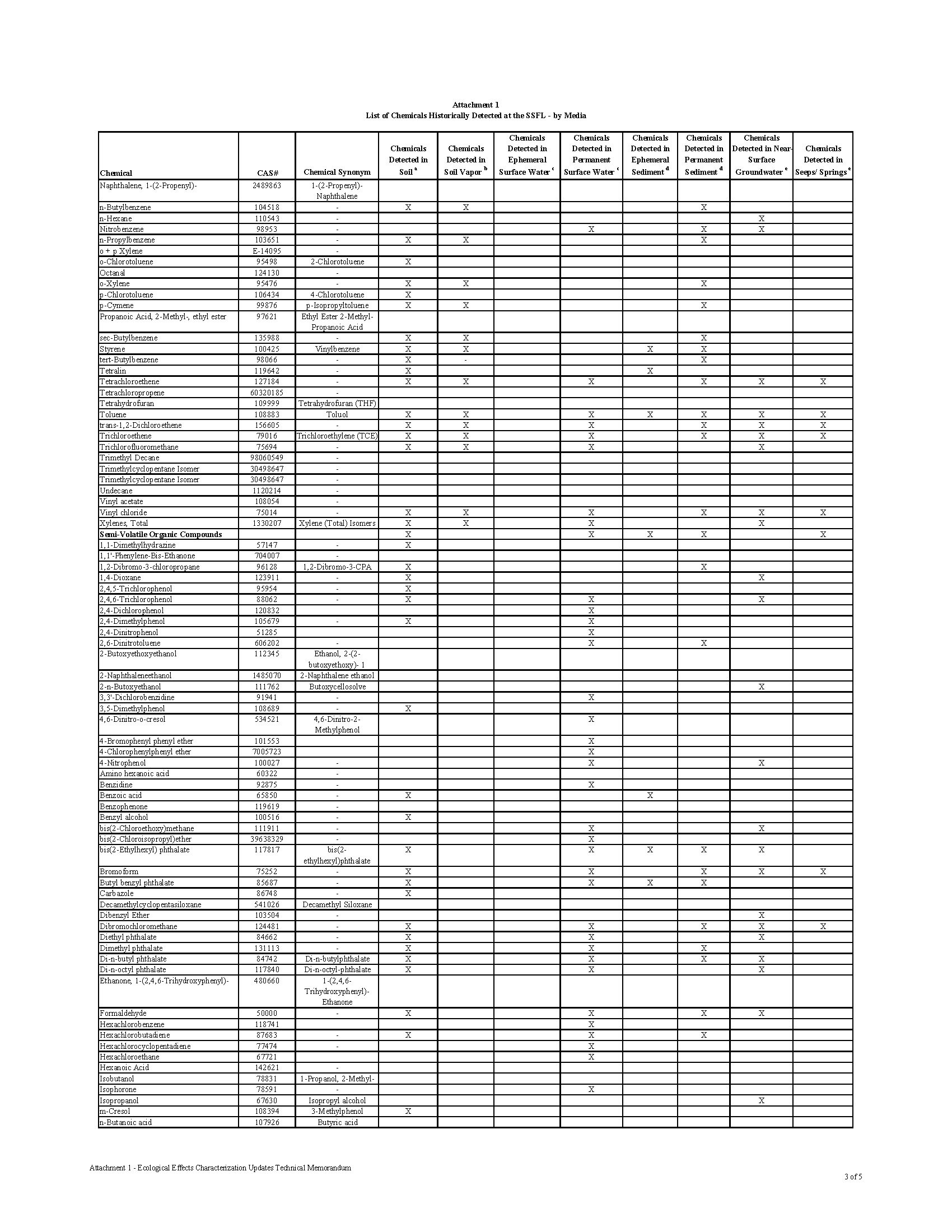
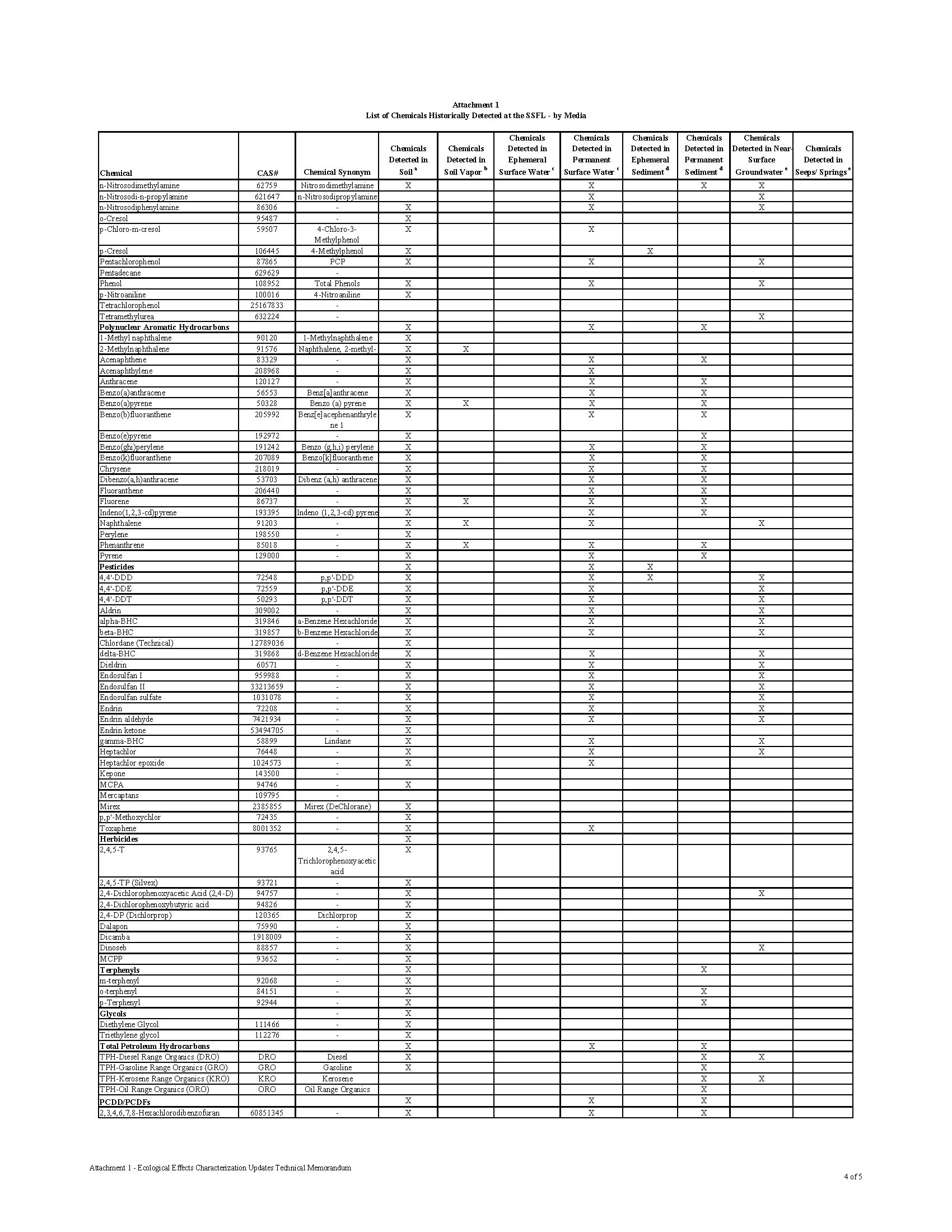
Over 300 Constituents of Concern (COCs) have been identified in the soil and groundwater at the Santa Susana Field Lab (SSFL), including toxic chemicals, heavy metals, and radioactive waste.
While this number is staggering, it is not surprising given the decades of experimental work conducted at the site. However, despite the well-documented contamination, the U.S. Centers for Disease Control and Prevention (CDC) and the National Cancer Institute (NCI) do not classify the numerous cancer cases near SSFL as a “cancer cluster.” To receive this designation, cases must involve the same or nearly identical type of cancer occurring at higher-than-expected rates in a specific community.
The issue is that most toxic sites do not contain the extreme variety of contaminants found at SSFL. Given the overwhelming range of carcinogens and toxins, it is no surprise that so many residents suffer from various types of cancer and other serious illnesses. The real problem lies in the fact that federal agencies like the CDC and NCI are not equipped to properly assess or address contamination sites as complex as SSFL, leaving affected communities without the recognition or support they deserve.
The contamination at the Santa Susana Field Lab is as diverse as it is dangerous. The site is rank with radioactive, chemical and heavy metal contamination. What makes the contamination so dangerous to the public is that the contamination isn’t buried in barrels underground. It was released into the environment through numerous accidents, open-air fires, spills, leaks, and explosions. The contamination is mixed in the soil and groundwater, making it easy for the contamination to migrate into the communities downhill.
Chemical waste generated at the SSFL facility was treated and stored on site, including in 28 surface ponds designed to collect cooling and rinse water, storm water runoff, and accidental spills. Many of these areas may have lacked proper containment facilities to prevent release of contaminants to the environment in the event of improper storage or spills throughout their operation. The Potential for Offsite Exposures Associated with Santa Susana Field Laboratory, Ventura County, California, Page 7
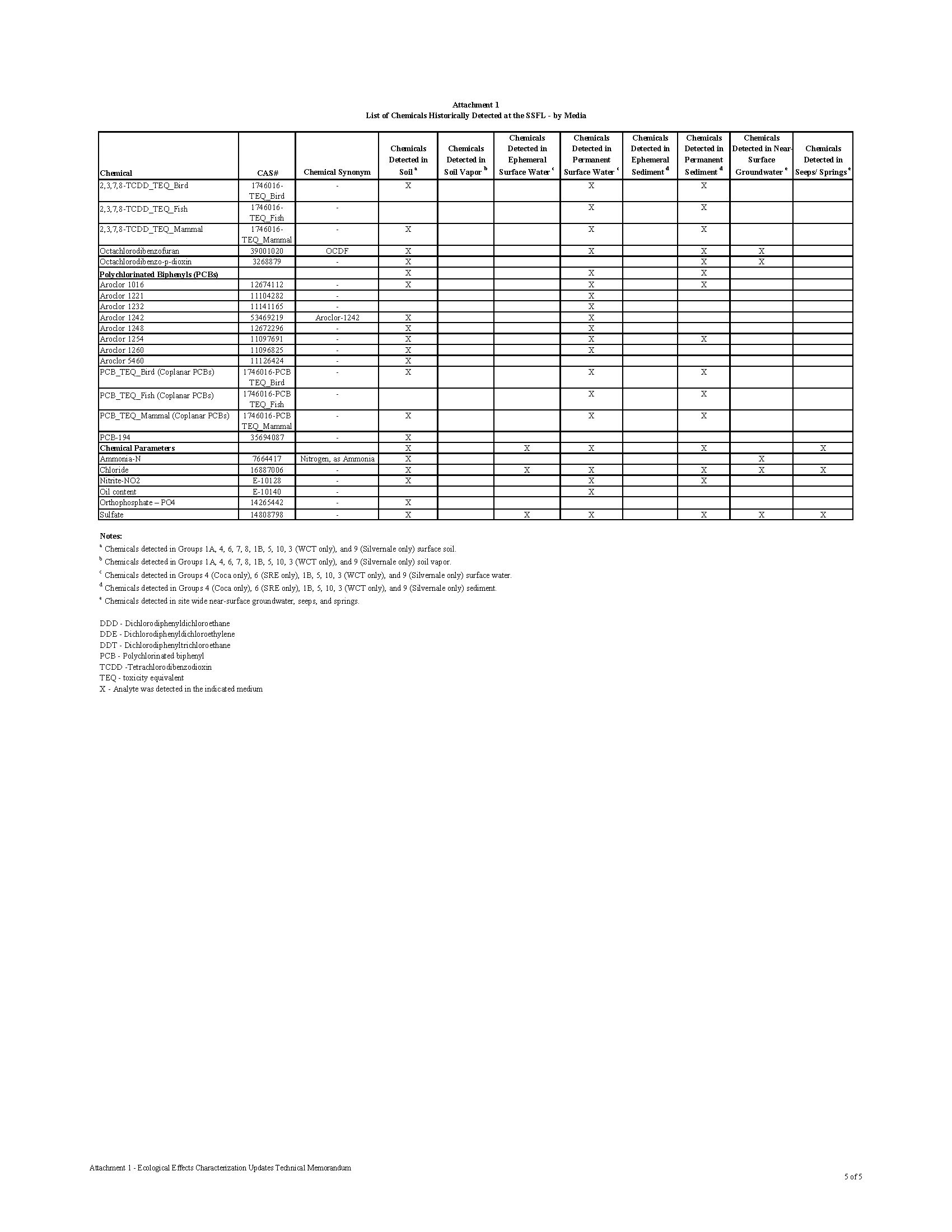
CHEMICAL CONTAMINATION
RADIOACTIVE CONTAMINATION
Some of the radionuclides of concern at the SSFL include:
Cesium-137: Multiple Radiation Emitter. Exposure to Cs-137 can increase the risk for cancer because of the presence of high-energy gamma radiation. Internal exposure to Cs-137 through ingestion or inhalation allows the radioactive material to be distributed in the soft tissues, especially muscle tissue, which increases cancer risk. Half-life is 30 years, hazardous-life is 600 years.
Strontium-90: Beta Emitter. Strontium-90 can be inhaled, but ingestion in food and water is the greatest health concern. Once in the body, Sr-90 acts like calcium and is readily incorporated into bones and teeth, where it can cause cancers of the bone, bone marrow, and soft tissues around the bone. Half-life is 29 years, hazardous-life is 580 years.
Plutonium-239: Alpha emitter. Low-energy x-ray emitter. Easily absorbed by tissue. Human exposure occurs mainly by breathing contaminated air or ingesting contaminated food or drink. When plutonium particles are inhaled and lodge in lung tissue, they continue to give off radiation internally. They can remain in the lungs or enter the gastrointestinal tract and the bloodstream. About 80 percent of the plutonium that enters the bloodstream goes either to the liver, bone or bone marrow, where it is retained for years, damaging tissue nearby. That damage may later develop into cancer. The half-life of plutonium-239 is about 24,000 years. Hazardous-life is 482,000 years.
"A few millionths of an ounce of plutonium-239, if inhaled, will result in cancer with a virtual 100% statistical certainty."
(pdf p. 6) “The Hazard from Plutonium Dispersal by Nuclear-warhead Accidents,” by Steve Fetter and Frank von Hippel, in Science and Global Security, Vol. 2, No. 1 (1990), pp. 21–41. (0.08 micrograms = ~3 millionths of an ounce).
Thorium: There are natural and man-made forms of thorium, all of which are radioactive. If inhaled as dust, some thorium may remain in the lungs for long periods of time, depending on the chemical form. There is research evidence that inhaling thorium dust increases the risk of lung and pancreatic cancer. Individuals exposed to thorium also have an increased risk of bone cancer because thorium may be stored in bone. Thorium may also be linked to brain cancer, specifically glioblastoma and DIPG.
Tritium: Beta Emitter. Cancer is the main risk from humans ingesting tritium. When tritium decays it spits out a low-energy electron (roughly 18,000 electron volts) that escapes and slams into DNA, a ribosome or some other biologically important molecule. And, unlike other radionuclides, tritium is usually part of water, so it ends up in all parts of the body and therefore can, in theory, promote any kind of cancer. But that also helps reduce the risk: any tritiated water is typically excreted in less than a month.
Uranium-235, 238: Primarily alpha emitter; low-energy gamma and beta particles are also emitted, depending on the isotope. Once in the body, uranium can accumulate in bones and kidneys. Some isotopes of uranium (e.g., U-235) are fissile and have applications in nuclear reactors and weapons. Prolonged exposure to enriched uranium’s radiation increases the risk of lung, bone, and kidney cancers. Additionally, uranium dust inhalation has been linked to respiratory issues and increased cancer risk. Uranium-238 has a half-life of about 4.5 billion years, and uranium-235 has a half-life of 700 million years. Due to these incredibly long half-lives, uranium remains hazardous essentially indefinitely, particularly when concentrated.
REFERENCES:
PCBs and PFAS
2021 GAO report (which covers Santa Susanna and talks generally about PFAS: https://www.gao.gov/assets/gao-21-205.pdf
Superfund sites with PFAS contamination: https://www.epw.senate.gov/public/index.cfm/superfund-sites-identified-by-epa-to-have-pfas-contamination
TCE and PFAS comingled in groundwater plumes is not at all unusual. At NASA’s Wallops Island facility in Virginia, for example, they have found both. Here are a couple of links that discuss what’s being done there:






